Bozeman science video questions molecules of life answer key – Embark on a scientific odyssey with the Bozeman Science video questions and answer key on the fundamental building blocks of life: molecules. This comprehensive guide unlocks the intricacies of molecular structure, properties, and their significance in biological systems, empowering you with a deeper understanding of the microscopic foundations of life on Earth.
Delve into the fascinating realm of water’s molecular architecture, unraveling its polarity and hydrogen bonding network. Discover how these unique properties endow water with remarkable surface tension and specific heat capacity, making it an indispensable solvent and a crucial component of life’s processes.
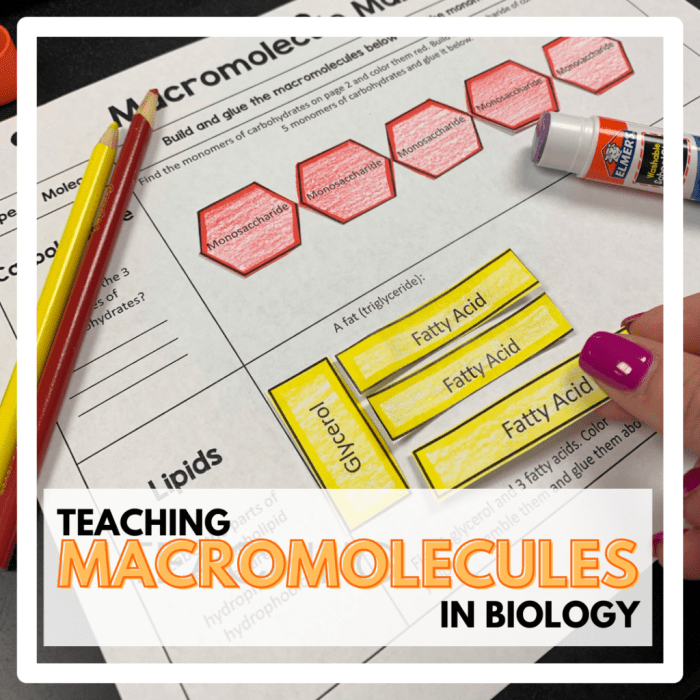
Explore the diverse roles of biological molecules – carbohydrates, lipids, proteins, and nucleic acids – and their intricate functions within living organisms.
Molecular Structure
The molecular structure of a substance refers to the arrangement of its constituent atoms and the chemical bonds that hold them together.
Water
Water (H2O) is a polar molecule, meaning it has a positive end and a negative end. The polarity of water molecules arises from the uneven distribution of electrons between the oxygen and hydrogen atoms. The oxygen atom has a higher electronegativity than the hydrogen atoms, meaning it attracts electrons more strongly.
This results in a partial negative charge on the oxygen atom and partial positive charges on the hydrogen atoms.
The polarity of water molecules allows them to form hydrogen bonds with each other. Hydrogen bonds are intermolecular forces that occur between a hydrogen atom bonded to an electronegative atom (such as oxygen, nitrogen, or fluorine) and another electronegative atom.
The hydrogen atom in the hydrogen bond is partially positive, and the electronegative atom is partially negative. This partial positive charge on the hydrogen atom is attracted to the partial negative charge on the electronegative atom, forming a hydrogen bond.
The hydrogen bonding network in water is responsible for many of its unique properties, such as its high surface tension and specific heat capacity.
Carbon Dioxide
Carbon dioxide (CO2) is a nonpolar molecule. The carbon atom is surrounded by two oxygen atoms, and the electrons are evenly distributed around the molecule. This even distribution of electrons results in no net polarity.
Methane, Bozeman science video questions molecules of life answer key
Methane (CH4) is a nonpolar molecule. The carbon atom is surrounded by four hydrogen atoms, and the electrons are evenly distributed around the molecule. This even distribution of electrons results in no net polarity.
| Molecule | Polarity | Hydrogen Bonding |
|---|---|---|
| Water (H2O) | Polar | Yes |
| Carbon Dioxide (CO2) | Nonpolar | No |
| Methane (CH4) | Nonpolar | No |
[Diagram of the hydrogen bonding network in water]
Expert Answers: Bozeman Science Video Questions Molecules Of Life Answer Key
What is the significance of hydrogen bonding in water?
Hydrogen bonding in water molecules grants it unique properties, including high surface tension and specific heat capacity, making it an essential solvent and a crucial component of life’s processes.
How do biological molecules contribute to living organisms?
Biological molecules, such as carbohydrates, lipids, proteins, and nucleic acids, play diverse roles in living organisms, including energy storage, membrane formation, enzyme catalysis, and genetic information storage.
What are macromolecules, and why are they important?
Macromolecules are large, complex molecules that perform essential functions in biological systems. They include cellulose, starch, and collagen, and are synthesized and broken down through intricate chemical reactions catalyzed by enzymes.