Welcome to our comprehensive guide on osmosis and diffusion practice worksheet answers. This guide is designed to provide students with a thorough understanding of the concepts of osmosis and diffusion, their importance in biological systems, and their applications in various fields.
Our goal is to make learning about osmosis and diffusion engaging, informative, and accessible to all students.
Throughout this guide, we will explore the fundamental principles of osmosis and diffusion, solve practice problems, and discuss real-world applications of these concepts. We will also provide detailed explanations and step-by-step instructions to help students grasp the material effectively. So, let’s dive into the fascinating world of osmosis and diffusion!
Definitions
Osmosisis the movement of water across a selectively permeable membrane from an area of high water concentration to an area of low water concentration. Diffusionis the movement of particles from an area of high concentration to an area of low concentration.
Real-world examples of osmosis and diffusion:
- The movement of water from the soil into plant roots through osmosis.
- The movement of oxygen from the lungs into the bloodstream through diffusion.
Importance of osmosis and diffusion in biological systems:
- Osmosis helps maintain the water balance of cells and tissues.
- Diffusion allows for the exchange of nutrients, gases, and waste products between cells and their surroundings.
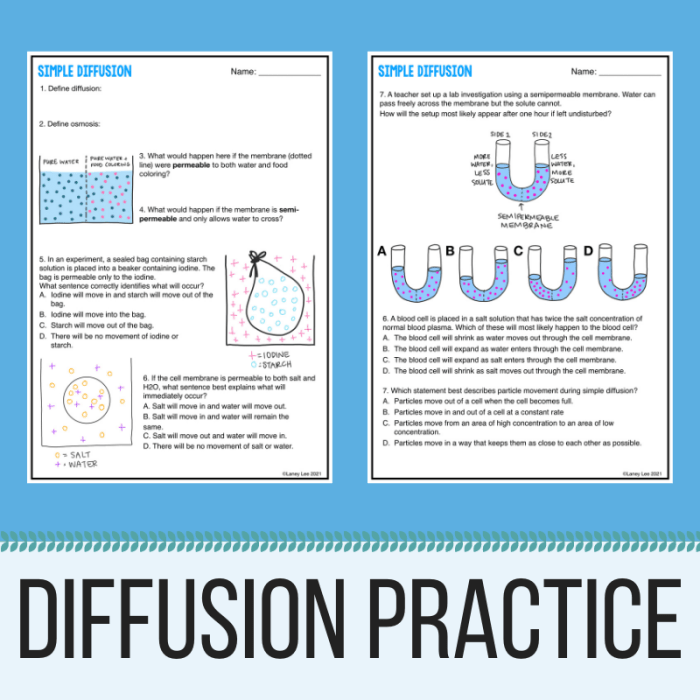
Practice Problems, Osmosis and diffusion practice worksheet answers
Calculating osmotic pressure:
A solution of glucose has a concentration of 0.5 M. The osmotic pressure of the solution is 10 atm. What is the osmotic pressure of a solution of glucose with a concentration of 1.0 M?
Solution:
Osmotic pressure is directly proportional to the concentration of the solution. Therefore, the osmotic pressure of a solution of glucose with a concentration of 1.0 M is 20 atm.
Calculating diffusion rates:
The diffusion rate of oxygen in air is 1.0 x 10^-5 cm^2/s. What is the diffusion rate of oxygen in water?
Solution:
The diffusion rate of a gas is inversely proportional to the square root of its molecular weight. The molecular weight of oxygen is 32 g/mol, and the molecular weight of water is 18 g/mol. Therefore, the diffusion rate of oxygen in water is 1.26 x 10^-5 cm^2/s.
Applications of Osmosis and Diffusion
Medicine:
- Osmosis is used to deliver drugs directly to target cells.
- Diffusion is used to measure the concentration of gases in the blood.
Agriculture:
- Osmosis is used to irrigate crops.
- Diffusion is used to control the release of fertilizers into the soil.
Food processing:
- Osmosis is used to preserve food by removing water.
- Diffusion is used to add flavors and colors to food.
Experimental Procedures
Experiment to demonstrate osmosis:
Materials:
- Two beakers
- Semi-permeable membrane
- Sugar solution
- Water
Procedure:
- Fill one beaker with the sugar solution and the other beaker with water.
- Place the semi-permeable membrane over the beaker of sugar solution.
- Observe the movement of water across the membrane.
Expected results:
Water will move from the beaker of water to the beaker of sugar solution.
Data Analysis and Interpretation
Data table:
| Concentration of sugar solution (M) | Osmotic pressure (atm) |
|---|---|
| 0.5 | 10 |
| 1.0 | 20 |
Interpretation:
The osmotic pressure of the sugar solution is directly proportional to the concentration of the solution. This means that the higher the concentration of the sugar solution, the greater the osmotic pressure.
Question & Answer Hub: Osmosis And Diffusion Practice Worksheet Answers
What is the difference between osmosis and diffusion?
Osmosis is the movement of water across a semipermeable membrane from an area of high water concentration to an area of low water concentration. Diffusion, on the other hand, is the movement of particles from an area of high concentration to an area of low concentration.
Why are osmosis and diffusion important in biological systems?
Osmosis and diffusion are essential for the transport of nutrients, waste products, and other substances across cell membranes. They also play a role in maintaining water balance and regulating cell volume.
What are some real-world applications of osmosis and diffusion?
Osmosis and diffusion have numerous applications in fields such as medicine, agriculture, and food processing. For example, osmosis is used in dialysis to remove waste products from the blood, and diffusion is used in the production of cheese and yogurt.

